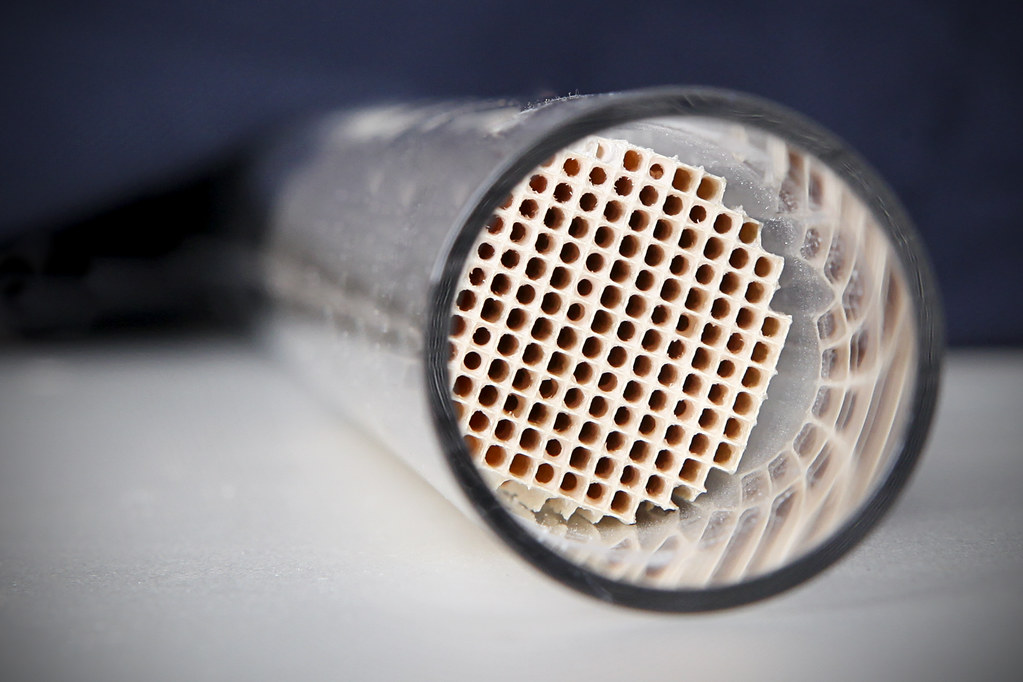
- Materials Used to Make Catalytic Converters
- How Do Catalytic Converters Work
- Benefits of Using a Catalytic Converter
- Chemistry Behind Catalytic Converters
- Different Types of Catalytic Converters
- Pros & Cons of Using a Ceramic-Based Catalyst
- Platinum in a High-Performance Converter
- Comparing Metal and Ceramic Substrates
- How Temperature Affects the Performance
- Automotive Emissions Regulations
Exploring the Different Materials Used to Make Catalytic Converters
Catalytic converters are an essential component of modern vehicles, as they help reduce the number of harmful emissions released into the environment. As such, it is important to understand the different materials used to make catalytic converters.
- The most common material used in catalytic converters is a ceramic honeycomb substrate. This substrate is made from a combination of alumina and other metals such as palladium, platinum, and rhodium. The honeycomb structure provides a large surface area for the catalyst to react with exhaust gases, allowing for a more efficient conversion of pollutants into less harmful substances.
- In addition to ceramic substrates, metal substrates are also used in some catalytic converters. These substrates are typically made from stainless steel or titanium and provide greater durability than ceramic substrates while still providing adequate surface area for reaction with exhaust gases. Metal substrates also tend to be more cost-effective than ceramic ones due to their lower production costs and longer lifespan.
- Finally, some catalytic converters use precious metal-coated foam as their substrate material instead of either ceramics or metals. This type of substrate offers superior performance compared to other materials due to its high surface area-to-volume ratio and ability to withstand higher temperatures without degrading over time. However, this type of substrate is also much more expensive than either ceramics or metals due to the high cost associated with coating it with precious metals like platinum or palladium.
Overall, there are several different materials that can be used in catalytic converter construction depending on the desired performance characteristics and budget constraints associated with each project. To learn more, check out our guides on which catalytic converters have the most rhodium, as well as how much platinum is in a catalytic converter.
Ceramic honeycomb substrates offer good performance at an affordable price point while metal substrates provide greater durability but come at a higher cost compared to ceramics; finally, precious metal-coated foam offers superior performance but comes at an even higher price tag than either ceramics or metals alone.
How Do Catalytic Converters Work?
Catalytic converters are an important part of a vehicle’s exhaust system. They are designed to reduce the number of harmful pollutants released into the atmosphere by converting them into less harmful substances.
The catalytic converter works by using a catalyst, usually platinum or palladium, to speed up chemical reactions in the exhaust gases. These reactions convert carbon monoxide and hydrocarbons into carbon dioxide and water vapor, which are much less damaging to the environment.
The catalytic converter is made up of two chambers separated by a honeycomb-like structure coated with precious metals such as platinum or palladium. As exhaust gases pass through this structure, they come in contact with these metals which act as a catalyst for chemical reactions that break down pollutants into harmless compounds.
The first chamber oxidizes hydrocarbons and carbon monoxide while the second chamber reduces nitrogen oxide (NOx) emissions from diesel engines. The catalytic converter is an essential component of any vehicle’s emission control system and helps reduce air pollution levels significantly when functioning properly.
It is important to keep your car’s catalytic converter in good condition so it can continue to do its job effectively; regular maintenance such as changing spark plugs and filters can help ensure that your car’s emissions remain low over time. Moreover, when you consider the catalytic converter precious metal scrap prices.
The Benefits of Using a Catalytic Converter
A catalytic converter is an important component of a vehicle’s exhaust system. It is designed to reduce the number of harmful pollutants released into the atmosphere by converting them into less harmful substances. The use of catalytic converters has become increasingly important in recent years due to increasing environmental concerns and regulations.
- The primary benefit of using a catalytic converter is that it helps reduce air pollution. Catalytic converters work by converting toxic gases such as carbon monoxide, hydrocarbons, and nitrogen oxides into less harmful substances such as carbon dioxide, water vapor, and nitrogen gas. This process helps reduce the amount of these pollutants released into the atmosphere, which can have a positive impact on air quality and public health.
- In addition to reducing air pollution, using a catalytic converter can also help improve fuel efficiency. By reducing emissions from vehicles, they can run more efficiently which can lead to improved fuel economy over time. This can result in significant savings for drivers who use their vehicles regularly or for long distances.
- Finally, using a catalytic converter may also help extend the life of your vehicle’s engine by reducing wear on its components caused by excessive emissions from combustion processes within the engine itself. By helping keep these components clean and free from buildup over time, they can function more effectively which can lead to improved performance and longevity for your vehicle’s engine overall.
Overall, there are many benefits associated with using a catalytic converter in your vehicle’s exhaust system including reduced air pollution levels; improved fuel efficiency; and extended engine life due to reduced wear on its components caused by excessive emissions from combustion processes within the engine itself.
Understanding the Chemistry Behind Catalytic Converters
Catalytic converters are an essential component of modern automobiles, helping to reduce the number of harmful pollutants released into the atmosphere. Understanding the chemistry behind these devices is key to appreciating their importance and effectiveness.
At its core, a catalytic converter is a device that uses a chemical reaction to convert toxic exhaust gases into less harmful substances. The process involves three main components: an oxidation catalyst, a reduction catalyst, and an adsorption catalyst.
The oxidation catalyst is responsible for converting carbon monoxide (CO) and hydrocarbons (HC) into carbon dioxide (CO2) and water vapor (H2O). This reaction occurs when oxygen molecules in the exhaust gas react with CO and HC molecules at high temperatures. The result is two harmless byproducts: CO2 and H2O.
The reduction catalyst works in tandem with the oxidation catalyst to further reduce emissions by converting nitrogen oxides (NOx) into nitrogen gas (N2). This reaction occurs when NOx molecules react with hydrogen atoms at high temperatures, resulting in N2 as a byproduct.
Finally, the adsorption catalyst helps capture any remaining pollutants before they can escape out of the tailpipe. It does this by trapping particles on its surface until they can be burned off during normal engine operation or removed during periodic maintenance intervals.
By combining these three components in one device, catalytic converters can effectively reduce emissions from automobiles without sacrificing performance or fuel economy. As such, they have become an indispensable part of modern automotive technology—helping us keep our air clean while still enjoying all that our cars have to offer.
What Are the Different Types of Catalytic Converters?
Catalytic converters are an important part of a vehicle’s exhaust system, as they reduce the number of harmful pollutants released into the atmosphere. There are several different types of catalytic converters available, each designed to meet specific needs and requirements.
- The most common type is the three-way catalytic converter, which is designed to reduce emissions from gasoline engines. This type of converter uses a combination of platinum, palladium, and rhodium to convert carbon monoxide (CO) into carbon dioxide (CO2), hydrocarbons (HC) into the water, and nitrogen oxides (NOx) into nitrogen gas.
- Another type is the diesel oxidation catalyst (DOC), which is used in diesel engines to reduce emissions such as particulate matter and hydrocarbons. This type uses a combination of platinum and palladium to oxidize HCs and COs into CO2 and water vapor.
- The selective catalytic reduction (SCR) converter is used in diesel engines to reduce NOx emissions by converting them into harmless nitrogen gas using urea or ammonia as a reducing agent. The SCR also helps improve fuel economy by reducing engine backpressure (especially when paired with a high-flow catalytic converter) caused by NOx buildup in the exhaust system.
- Finally, there are lean NOx traps (LNTs), which use zeolite-based materials such as barium oxide or potassium oxide to trap NOx molecules until they can be converted back into harmless nitrogen gas through thermal regeneration or active regeneration with fuel additives like ethanol or methanol injection systems. LNTs are typically used in light-duty vehicles that have gasoline direct injection systems or turbocharged engines that produce higher levels of NOx than traditional gasoline engines do.
The Pros and Cons of Using a Ceramic-Based Catalyst in a Catalytic Converter
The use of a ceramic-based catalyst in a catalytic converter is becoming increasingly popular due to its ability to reduce harmful emissions from vehicles. This type of catalyst has several advantages and disadvantages that should be considered before making the decision to install one.
Pros:
1. Ceramic-based catalysts are highly efficient at reducing emissions, such as carbon monoxide, hydrocarbons, and nitrogen oxides. This makes them an ideal choice for those looking to reduce their vehicle’s environmental impact.
2. They are also more durable than other types of catalysts, meaning they will last longer and require less maintenance over time.
3. Ceramic-based catalysts can also help improve fuel economy by allowing engines to run more efficiently with less fuel consumption.
4. Finally, these types of catalysts are relatively inexpensive compared to other options on the market today, making them an attractive option for those on a budget who still want the benefits of reduced emissions from their vehicle’s exhaust system.
Cons:
1. One potential downside is that ceramic-based catalysts can be difficult to install due to their size and weight compared with other types of converters available today; this may require professional installation or additional modifications for it to fit properly into your vehicle’s exhaust system.
2. Additionally, these types of converters tend not to be as effective at reducing sulfur dioxide (SO2) emissions as some other options on the market, which could lead to higher levels of air pollution if not addressed properly.
3. Finally, ceramic-based converters may require more frequent replacement than some other options due to their shorter catalytic converter life expectancy; this could lead to increased costs over time if replacements become necessary.
In conclusion, while there are both pros and cons associated with using a ceramic-based catalyst in a catalytic converter, it is ultimately up to the individual consumer whether or not they choose this option for their vehicle’s exhaust system.
It is important that all factors be taken into consideration before making any decisions so that you can make an informed choice about what type of converter best suits your needs and budget.
Examining the Role of Platinum in Making a High-Performance Catalytic Converter
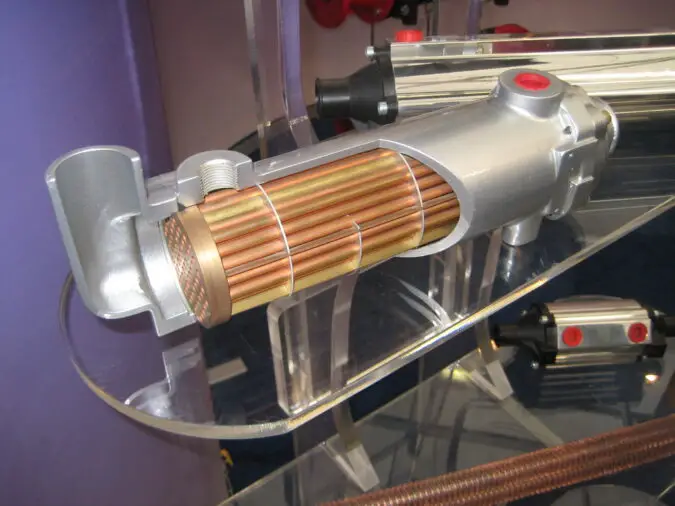
“Catalytic Converter” by Hiddenpower is licensed under CC BY-NC-SA 2.0
The catalytic converter is a vital component of modern automobiles, as it helps to reduce the number of harmful emissions released into the atmosphere. For a catalytic converter to be effective, it must be made from high-performance materials that can withstand extreme temperatures and pressures.
One such material is platinum, which has been used in catalytic converters since its introduction in 1975. Platinum is an ideal material for use in catalytic converters due to its unique properties. It has a high melting point and excellent corrosion resistance, making it highly durable and able to withstand extreme temperatures and pressures without degrading or corroding over time.
Additionally, platinum has excellent thermal conductivity properties which allow heat generated by the exhaust system to be quickly dissipated away from the catalyst surface. This helps ensure that the catalyst remains at an optimal temperature for efficient operation. You can confirm this with a catalytic converter temperature test.
Furthermore, platinum also acts as an effective catalyst itself due to its ability to form strong bonds with other elements such as oxygen and nitrogen oxides (NOx). This allows it to break down these pollutants into harmless compounds before they are released into the atmosphere.
As such, platinum plays an important role in helping reduce air pollution levels by converting harmful gases into less toxic substances before they are emitted from vehicle exhausts. In conclusion, platinum is essential for creating high-performance catalytic converters that can effectively reduce air pollution levels.
While also being able to withstand extreme temperatures and pressures without degrading or corroding over time. Its unique properties make it one of the most reliable materials available for use in this application today, ensuring that vehicles remain safe and environmentally friendly on our roads today.
Comparing Metal and Ceramic Substrates for Use in Making a Catalyst
Metal and ceramic substrates are both commonly used in the production of catalysts. Each material has its own unique advantages and disadvantages, making it important to consider the specific needs of a given application before selecting a substrate. This article will provide an overview of the differences between metal and ceramic substrates for use in making catalysts.
- Metal substrates are typically composed of metals such as nickel, iron, or cobalt. These materials have high thermal conductivity, which allows them to quickly transfer heat away from the catalyst during operation. Additionally, metal substrates can be easily machined into complex shapes that may be necessary for certain applications. However, metal substrates can corrode over time due to exposure to chemicals or other environmental factors, which can reduce their effectiveness as a catalyst substrate over time.
- Ceramic substrates are composed of materials such as alumina or silica-based ceramics that have been fired at high temperatures to create a dense material with excellent thermal stability and chemical resistance properties. Ceramic substrates also offer good mechanical strength and durability compared to metal counterparts; however, they tend to be more expensive than metal options due to their manufacturing process being more complex and labor intensive. Additionally, ceramic materials may not be able to withstand extreme temperatures or pressures that some applications require without cracking or breaking apart over time.
When selecting a substrate for use in making catalysts it is important to consider both the cost and performance requirements of the application at hand before deciding on either a metal or ceramic option.
Metal substrates offer good thermal conductivity but may corrode over time while ceramic options provide excellent chemical resistance but tend to be more expensive than their metallic counterparts due to their manufacturing process being more complex and labor-intensive.
Investigating How Temperature Affects the Performance of a Catalyst
Investigating the effects of temperature on the performance of a catalyst is an important area of research in chemical engineering. Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process.
They are used to reduce energy requirements and production costs, as well as to improve product quality and safety. Temperature is one factor that can affect how efficiently a catalyst works, so it is important to understand how different temperatures can influence its performance.
The activity of a catalyst depends on its ability to form strong bonds with reactants, which require energy input from heat or light. As temperature increases, more energy becomes available for these bonds to form and break apart, resulting in faster reaction rates.
However, if temperatures become too high, they can cause irreversible damage to the catalyst’s structure and reduce its effectiveness over time. Therefore, it is essential for researchers to identify an optimal temperature range for each type of catalyst to maximize efficiency while avoiding damage from excessive heat exposure.
In addition to affecting reaction rates directly through increased energy availability, temperature also affects catalysts indirectly by changing their physical properties such as solubility or surface area available for reactions. For example, some catalysts may become less soluble at higher temperatures which could limit their ability to interact with reactants and slow down reactions overall.
Similarly, increasing temperatures may cause some catalysts’ surface areas available for reactions (such as pores)to shrink or collapse due to thermal expansion or contraction which could also reduce their effectiveness over time if not monitored closely enough.
Overall, understanding how different temperatures affect the performance of catalysts is essential for optimizing processes involving them. By conducting experiments at various temperatures, researchers can determine optimal ranges where maximum efficiency can be achieved while minimizing potential damage caused by excessive heat exposure.
This knowledge will help ensure that processes using catalysts are optimized correctly, leading toward improved product quality, safety, cost savings, and environmental sustainability.
Analyzing How Automotive Emissions Regulations Impact the Design and Manufacturing Process
The automotive industry is subject to stringent regulations regarding emissions, and catalytic converters are a key component in helping vehicles meet these standards. As such, the design and manufacturing process for catalytic converters must be carefully considered to ensure that they can effectively reduce emissions.
This article will provide an overview of how automotive emissions regulations impact the design and manufacturing process for catalytic converters.
- To begin with, it is important to understand the role of a catalytic converter in reducing vehicle emissions. A catalytic converter works by converting harmful pollutants into less harmful substances before they are released into the atmosphere. This is accomplished through a chemical reaction between exhaust gases and a catalyst material inside the converter. The catalyst material typically consists of precious metals such as platinum, palladium, or rhodium which act as an oxidizing agents when exposed to exhaust gases at high temperatures.
- For a catalytic converter to be effective at reducing vehicle emissions, it must be designed according to specific requirements set forth by regulatory bodies such as the Environmental Protection Agency (EPA). These requirements include specifications on materials used in construction, the size of components within the converter, and overall efficiency ratings based on testing results from laboratory simulations or actual road tests. Additionally, manufacturers must adhere to strict quality control measures during production for their products to meet these standards.
- The design process for a new model of the catalytic converter begins with research into current regulations and trends within the industry so that engineers can develop solutions that meet all applicable requirements while also providing optimal performance levels under various driving conditions. Once this research has been completed, engineers will then create detailed designs using computer-aided drafting (CAD) software which can simulate real-world conditions so that any potential issues can be identified before production begins.
- Once designs have been finalized and approved by regulatory bodies such as EPA or CARB (California Air Resources Board), manufacturers will then begin producing parts according to those specifications using specialized machineries such as injection molding machines or CNC lathes/mills depending on what type of part needs to be produced (e.g., housing vs internal components). During this stage, it is important that quality control measures strictly adhere to since any defects could lead not only lead noncompliance with regulations but also to potentially dangerous situations if parts fail during operation due to improper construction/materials used, etc.
- Finally, once all parts have been produced they will need to undergo rigorous testing both in laboratory simulations as well as actual road tests before being approved for sale/installation onto vehicles; this ensures that all products meet the required standards set forth by regulatory bodies while also ensuring optimal performance levels under various driving conditions.
In conclusion, automotive emission regulations play an integral role in determining how catalytic converters should be designed and manufactured. By understanding these requirements, manufacturers can ensure their products comply with applicable laws while still providing optimal performance levels under various driving conditions.