- Materials Used In Catalytic Converters
- Using a Catalytic Converter Made of Platinum
- Catalytic Converter Made of Ceramic
- Role of Palladium in Catalytic Converters
- Why Is Rhodium Used in Catalytic Converters
- Durability and Efficiency of Different Types
- How Temperature Affects the Performance
- Automotive Emissions Regulations
- New Technologies That Could Improve
Exploring the Different Materials Used to Make Catalytic Converters
Catalytic converters are an essential component of modern vehicles, as they help reduce the number of harmful emissions released into the environment. As such, it is important to understand the different materials used to make catalytic converters. And, what is a catalytic converter made of…
- The most common material used in catalytic converters is a ceramic honeycomb substrate. This substrate is made from a combination of alumina and other metals such as palladium, platinum, and rhodium. The honeycomb structure provides a large surface area for the catalyst to react with exhaust gases, allowing for a more efficient conversion of pollutants into less harmful substances.
- In addition to ceramic substrates, metal substrates are also used in some catalytic converters. These substrates are typically made from stainless steel or titanium and provide greater durability than ceramic substrates while still providing adequate surface area for reaction with exhaust gases. Metal substrates also tend to be more cost-effective than ceramic ones due to their lower production costs and longer lifespan.
- Finally, some catalytic converters use precious metal-coated foam as their substrate material instead of either ceramics or metals. This type of substrate offers superior performance compared to other materials due to its high surface area-to-volume ratio and ability to withstand higher temperatures without degrading over time. However, this type of substrate is also much more expensive than either ceramics or metals due to the high cost associated with coating it with precious metals like platinum or palladium.
Overall, several different materials can be used in catalytic converter construction depending on the desired performance characteristics and budget constraints involved in each application scenario. To learn more, check out our explainer on which catalytic converters have the most rhodium, as well as how much platinum is in a catalytic converter.
Ceramic honeycomb substrates offer good performance at an affordable price point while metal substrates provide greater durability but come at a higher cost compared to ceramics; finally, precious metal-coated foam offers superior performance but comes at an even higher price tag than either ceramics or metals alone.
The Benefits of Using a Catalytic Converter Made of Platinum
A catalytic converter made of platinum is a highly efficient device used to reduce the number of harmful emissions released into the atmosphere. This type of converter is designed to convert toxic exhaust gases, such as carbon monoxide and hydrocarbons, into less harmful substances.
Platinum catalytic converters are widely used in automobiles and other vehicles due to their superior performance and durability. For more insight, check out our guide on what’s in catalytic converters.
- The primary benefit of using a platinum catalytic converter is its ability to reduce emissions more effectively than other types of converters. Platinum has a higher oxidation potential than other metals, which means it can break down pollutants more quickly and efficiently. This results in fewer pollutants being released into the environment, helping to improve air quality and reduce health risks associated with air pollution. Additionally, platinum catalytic converters can withstand higher temperatures than other types of converters, making them ideal for use in high-performance engines that generate large amounts of heat during operation.
- Another advantage offered by platinum catalytic converters is their long lifespan compared to other types of converters. Platinum has excellent corrosion resistance properties which allow it to last longer without needing replacement or repair work done on it. This helps save money over time since you won’t have to replace your converter as often as you would with another type of material such as stainless steel or aluminum alloy.
- Finally, using a platinum catalytic converter also helps protect the environment by reducing greenhouse gas emissions from vehicles that use them. By reducing these emissions, we can help slow down global warming and climate change while also improving air quality for everyone around us who breathes in this polluted air every day.
In conclusion, there are many benefits associated with using a platinum catalytic converter for your vehicle’s exhaust system including improved emission reduction efficiency, increased durability and lifespan compared with other materials used for this purpose as well as environmental protection through reduced greenhouse gas emissions from vehicles that use them regularly.
How Does a Catalytic Converter Made of Ceramic Work?
A catalytic converter made of ceramic is an important component of a vehicle’s exhaust system. It works by converting harmful pollutants in the exhaust gases into less harmful substances before they are released into the atmosphere.
The ceramic material used in catalytic converters is composed of several different metals, including platinum, palladium, and rhodium. These metals act as a catalyst to speed up the chemical reaction that takes place when the exhaust gases pass through them.
The process begins when the exhaust gases enter the converter and come into contact with these metal catalysts. The heat from these gases causes a chemical reaction between them and the oxygen molecules to present in the air.
This reaction produces carbon dioxide (CO2) and water vapor (H2O). The CO2 is then released out of the tailpipe while H2O condenses on its walls and eventually evaporates away. In addition to reducing emissions, this process also helps reduce engine noise levels by absorbing some of it before it exits through the tailpipe.
Catalytic converters made from ceramic materials are highly efficient at reducing emissions because they can withstand higher temperatures than other types of converters without breaking down or becoming damaged over time. You can confirm as much with a catalytic converter temperature test.
They also last longer than other types due to their durability and resistance to corrosion caused by exposure to high temperatures or chemicals found in gasoline or diesel fuel fumes. As such, they are an important part of any vehicle’s emission control system as they help reduce air pollution levels significantly while still allowing for optimal engine performance levels at all times.
Understanding the Role of Palladium in Catalytic Converters
Palladium is a precious metal that plays an important role in catalytic converters, which are devices used to reduce the number of harmful pollutants emitted from vehicles. Catalytic converters work by converting toxic gases such as carbon monoxide and hydrocarbons into less harmful substances like carbon dioxide and water vapor.
Palladium is used in catalytic converters because it can act as a catalyst, meaning it can speed up chemical reactions without being consumed itself. The palladium in catalytic converters is usually found in the form of a honeycomb-shaped ceramic substrate coated with a thin layer of palladium particles.
This substrate acts as a support for the palladium particles, allowing them to come into contact with exhaust gases passing through the converter. The palladium then acts as a catalyst for chemical reactions that convert toxic gases into less harmful substances before they are released into the atmosphere.
In addition to its role as a catalyst, palladium also helps reduce emissions by trapping unburned hydrocarbons and other pollutants before they can escape from vehicle exhaust systems. Also, if you’re thinking of scrapping your car’s catalytic converter for its precious metals, check out our guide on the catalytic converter precious metal scrap prices.
This process occurs when molecules of unburned fuel react with oxygen molecules on the surface of the palladium particles, forming compounds that are trapped within its structure until they can be broken down further by other catalysts inside the converter.
Overall, palladium plays an essential role in reducing emissions from vehicles by acting both as a catalyst and trap for pollutants inside catalytic converters. Without this precious metal, our air quality would suffer significantly due to increased levels of dangerous toxins being released into our environment every day from vehicle exhausts.
What Is Rhodium and Why Is It Used in Catalytic Converters?
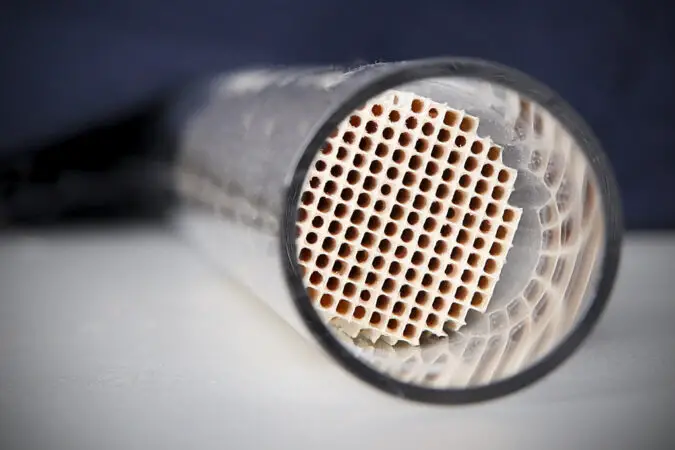
“Catalytic converter” by oakridgelabnews is licensed under CC BY 2.0
Rhodium is a rare, silvery-white metal that belongs to the platinum group of metals. It is highly resistant to corrosion and oxidation, making it an ideal material for use in catalytic converters. Catalytic converters are devices used in automobiles to reduce harmful emissions from the exhaust system.
They work by converting toxic gases such as carbon monoxide and hydrocarbons into less harmful substances like carbon dioxide and water vapor. Rhodium is used as a catalyst in catalytic converters because of its ability to absorb oxygen molecules from the exhaust gas stream.
This helps convert toxic gases into harmless ones more efficiently than other materials, such as palladium or platinum. Rhodium also has a high melting point which makes it suitable for use at high temperatures found in automobile engines. Additionally, rhodium does not corrode or degrade over time like other metals, so it can be used for many years without needing replacement.
Overall, rhodium is an ideal material for use in catalytic converters due to its resistance to corrosion and oxidation, its ability to absorb oxygen molecules from the exhaust gas stream, and its high melting point which allows it to withstand extreme temperatures found inside automobile engines.
Comparing the Durability and Efficiency of Different Types of Catalytic Converters
Catalytic converters are an essential component of modern vehicles, as they help reduce the number of harmful emissions released into the atmosphere. As such, it is important to consider the durability and efficiency of different types of catalytic converters when making a purchase.
This article will compare the durability and efficiency of three common types of catalytic converters: ceramic, metallic, and honeycomb.
- Ceramic catalytic converters are made from a combination of ceramic materials and precious metals such as platinum or palladium. They are known for their high efficiency in reducing emissions but can be prone to damage due to their fragile nature. Ceramic catalytic converters typically last between 50,000-100,000 miles before needing replacement.
- Metallic catalytic converters are made from stainless steel or other metal alloys that contain precious metals such as platinum or palladium. They offer excellent durability compared to ceramic models but may not be as efficient in reducing emissions due to their lower surface area for reaction with exhaust gases. Metallic catalytic converters typically last between 100,000-150,000 miles before needing replacement. So, be wary of the life expectancy of a catalytic converter.
- Honeycomb catalytic converters feature a honeycomb structure that increases surface area for reaction with exhaust gases while also providing greater durability than ceramic models due to their metal construction. Honeycomb models offer excellent efficiency in reducing emissions but may not last as long as metallic models due to their higher reactivity with exhaust gases; they typically need replacing after 80,000-120,000 miles depending on usage conditions and driving habits.
In conclusion, each type of catalytic converter offers its own advantages when it comes to durability and efficiency; however, it is important to consider your individual needs when making a purchase decision so you can get the most out of your investment over time.
Investigating How Temperature Affects the Performance of a Catalytic Converter Made Of Metal Alloys
Investigating the effects of temperature on the performance of a catalytic converter made of metal alloys is an important area of research. Catalytic converters are used in automobiles to reduce emissions and improve air quality.
The performance of these devices is largely dependent on the materials used in their construction, as well as the operating temperature. This article will discuss how temperature affects the performance of a catalytic converter made from metal alloys.
The primary function of a catalytic converter is to convert harmful pollutants into less toxic substances before they are released into the atmosphere. To do this, it relies on chemical reactions that occur between its components and exhaust gases passing through it.
These reactions require heat energy, which can be provided by either an external source or generated internally by oxidation processes within the device itself. As such, higher temperatures can lead to increased reaction rates and improved efficiency for these devices.
However, too much heat can also have detrimental effects on a catalytic converter’s performance due to thermal expansion and contraction caused by changes in temperature. This can cause warping or cracking in some parts, leading to reduced efficiency or even complete failure if left unchecked for too long.
Additionally, certain metals used in these devices may become brittle at high temperatures and lose their ability to withstand mechanical stress over time due to fatigue failure caused by repeated heating and cooling cycles over extended periods of use.
In conclusion, it is clear that temperature plays an important role in determining how well a catalytic converter performs its intended function when constructed from metal alloys. While higher temperatures may lead to increased reaction rates within these devices initially, prolonged exposure could eventually cause damage due to thermal expansion/contraction or fatigue failure resulting from repeated heating/cooling cycles over time if not properly monitored and managed accordingly.
Analyzing How Automotive Emissions Regulations Impact the Design and Materials Used
The automotive industry is subject to stringent regulations regarding emissions, and catalytic converters are a key component in helping vehicles meet these standards. As such, the design and materials used for making catalytic converters must be carefully considered to ensure that they are effective at reducing emissions.
The primary purpose of a catalytic converter is to reduce harmful exhaust gases from entering the atmosphere. This is achieved by using a catalyst material that reacts with the exhaust gases, converting them into less harmful substances before they are released into the environment.
The most common catalyst material used in modern vehicles is platinum-based, although other metals such as palladium and rhodium can also be used. To meet increasingly stringent emissions regulations, automotive manufacturers must use higher concentrations of these precious metals in their catalytic converters.
This increases their cost but also ensures that they are more effective at reducing emissions levels from vehicles. Additionally, manufacturers must also consider other design factors when creating their converters such as the size and shape of the converter body as well as its internal structure which affects how efficiently it can process exhaust gases.
In conclusion, automotive emission regulations have had a significant impact on the design and materials used for making catalytic converters over recent years. By using higher concentrations of precious metals such as platinum and palladium along with careful consideration of other design factors, manufacturers can ensure that their converters can effectively reduce vehicle emissions levels while still remaining cost-effective solutions for consumers.
Exploring New Technologies That Could Improve The Efficiency Of A Catalyst-Made Converter
Catalysts are essential components of many industrial processes, as they can help to increase the efficiency of a converter. As technology advances, new technologies are being developed that could further improve the efficiency of catalyst-made converters.
This article will explore some of these new technologies and their potential applications in improving the efficiency of catalytic converters.
- One such technology is nanotechnology, which involves manipulating matter on an atomic or molecular scale. Nanoparticles can be used to create catalysts with higher surface area and more active sites than traditional catalysts, allowing for faster reaction rates and improved conversion efficiencies. Additionally, nanomaterials can be tailored to specific reactions by altering their size and shape, allowing for greater control over the reaction process.
- Another promising technology is artificial intelligence (AI). AI algorithms can be used to optimize catalyst design by predicting how different parameters will affect reaction rates and conversion efficiencies. This allows researchers to quickly identify optimal conditions for a given reaction without having to conduct extensive experiments in a laboratory setting. AI-based optimization techniques have already been applied successfully in several areas related to catalysis research, including catalyst synthesis and screening methods as well as kinetic modeling approaches.
- Finally, 3D printing has emerged as another promising technology that could improve the efficiency of catalytic converters. 3D printing allows researchers to rapidly prototype complex structures with intricate geometries that would otherwise be difficult or impossible to fabricate using traditional manufacturing methods. These structures can then be tested under various conditions to determine their effectiveness at increasing conversion efficiencies or reducing emissions from exhaust gases produced during combustion processes involving catalytic converters.
In conclusion, there are several new technologies that have potential applications in improving the efficiency of catalyst-made converters. Nanotechnology offers increased control over reaction rates through tailored nanoparticles.
AI algorithms allow for rapid optimization of catalyst design, and 3D printing enables rapid prototyping of complex geometries for testing under various conditions related to catalysis research projects involving converter systems. With continued development and refinement these technologies may soon become integral components in improving the performance of industrial processes relying on catalytic converters.

